Abstract
Introduction
Hbb th1/th1 mice lack functional beta-globin, leading to decreased hemoglobin (Hb) production, decreased mature red blood cells (RBCs), and ineffective erythropoiesis. The model mimics several of the pathological changes seen in beta-thalassemia. IMR-687 (tovinontrine) is a highly selective phosphodiesterase-9 (PDE9) inhibitor which increases intracellular cGMP. The aim of this study was to assess the effect of IMR-687 on markers of beta-thalassemia in the Hbb th1/th1 mouse model.
Methods
Eighteen Hbb th1/th1 mice were divided into three groups of six animals each and dosed once daily, by gavage, for 30 days with IMR-687 at 30 or 60 mg/kg/day or vehicle control. On day 30, blood was collected for routine hematology including measurement of Hb, reticulocytes and RBCs, and spleen tissue was dissociated to assess erythrocyte differentiation using flow cytometry analysis of immature (Ery.B: Ter119 highCD71 highFSC low) and mature (Ery.C: Ter119 highCD71 lowFSC low) erythroblast populations. Animals were observed daily for mortality and measured for total body weight (Day 0 and Day 30) and spleen to body weight ratio at necropsy (Day 30).
Results
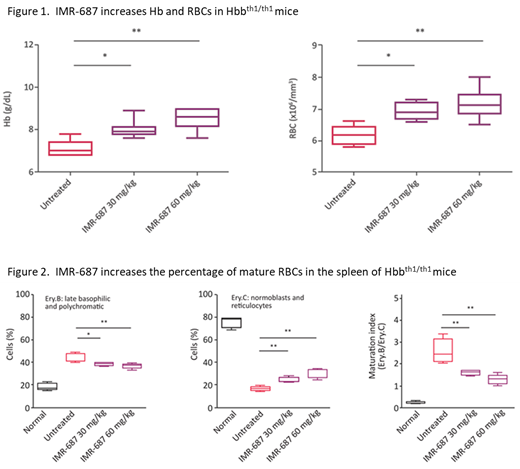
After 30 days of treatment, the groups of mice treated with IMR-687 at 30 or 60 mg/kg/day showed a mean increase in Hb of 1.0 g/dL (p<0.05) and 1.5 g/dL (p<0.01), respectively, relative to vehicle controls. RBCs showed a statistically significant increase (p<0.05) following treatment with IMR-687 at 30 or 60 mg/kg/day (6.9 and 7.2 x10 6 cells/ml, respectively) relative to vehicle (6.2 x10 6 cells/ml). The number of reticulocytes decreased after treatment with IMR-687 (only in the 30 mg/kg/day group, 1.8 x10 6 cells/ml, p<0.05) relative to vehicle (2.2 x10 6 cells/ml). Flow cytometry analysis of spleens showed that both doses of IMR-687 increased (p<0.05) the percentage of Ery.C cells (24.2% and 28.5% in 30 and 60 mg/kg/day, respectively) relative to vehicle (16.6%,) and decreased (p<0.05) the percentage of Ery.B cells (38.4% and 36.9% in 30 and 60 mg/kg/day, respectively) relative to vehicle (43.5%), resulting in a maturation ratio (Ery.B/Ery.C) that favored mature RBCs. There were no deaths nor any significant differences in total body weight or spleen to body weight ratio in animals treated with IMR-687 relative to vehicle controls.
Conclusions
Administration of IMR-687 at 30 or 60 mg/kg/day for 30 days improved markers of disease progression in a mouse model of beta-thalassemia, as shown by a statistically significant increase in Hb and RBCs and decrease in reticulocytes as well as improved differentiation of splenic erythroblasts. Both dose levels of IMR-687 were well tolerated with no treatment-related deaths or abnormal clinical signs. These results support a role for IMR-687 in beta-thalassemia by enabling RBC maturation and improving ineffective erythropoiesis, key components in ameliorating disease pathology. Clinical testing of IMR-687 (up to 400 mg) as a once daily, oral tablet is currently ongoing in a Phase 2 study of patients with beta-thalassemia (NCT04411082).
Maciel: Imara Inc.: Research Funding. Carvalho: Imara Inc.: Research Funding. Rignault: Imara Inc.: Research Funding. Allali: Imara Inc.: Research Funding. OCain: Imara Inc.: Current Employment, Current equity holder in publicly-traded company. Ballal: Imara Inc.: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal